Description
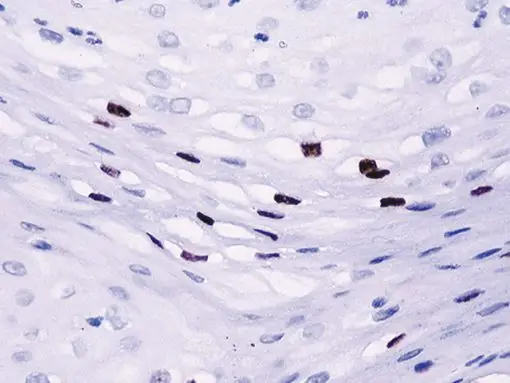
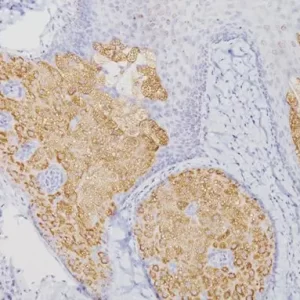
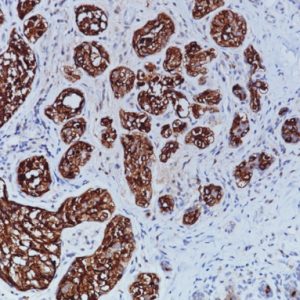
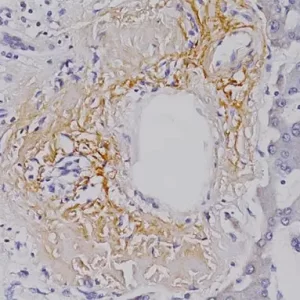
The CAMVIR-1 antibody was raised against the major capsid protein L1 of human papillomavirus HPV-16 antibody, using a recombinant vaccinia virus that expresses the L1 protein. This antibody also detects the HPV-16 L1 antigen in formalin-fixed, paraffin embedded biopsy specimens and on routine cervical smears. The antibody reacts strongly and consistently with specimens containing HPV-16 or HPV-33, but very weak reactions were occasionally observed with biopsy specimens or smears containing HPV-6 or HPV-11.
SPECIFICATIONS
Specifications
| INTENDED USE | RUO |
|---|---|
| SPECIES REACTIVITY | Human |
| SOURCE | Mouse Monoclonal |
| CLONE | CAMVIR-1 |
| ISOTYPE | IgG2a |
| ANTIGEN | HPV-16 |
| LOCALIZATION | Nuclear |
| POSITIVE CONTROL | Infected cervical biopsyNuclear |
DATASHEETS & SDS
OUS ONLY IVD
REFERENCES
1. McLean CS, Churcher MJ, Meinke J, Smith GL, Higgins G, Stanley M, Minson AC. Production and characterisation of a monoclonal antibody to human papillomavirus type 16 using recombinant vaccinia virus. J Clin Pathol 1990 Jun;43 (6):488-92.
2. Cowsert LM, Pilacinski WP, Jenson AB. Identification of the bovine papillomavirus L1 gene product using monoclonal Antibodies. Virology 1988 Aug;165(2):613
3. Center for Disease Control Manual. Guide: Safety Management, NO. CDC-22, Atlanta, GA. April 30, 1976 “Decontamination of Laboratory Sink Drains to Remove Azide Salts.”
4. National Committee for Clinical Laboratory Standards (NCCLS). Protection of laboratory workers from infectious diseases transmitted by blood and tissue; proposed guideline. Villanova, PA 1991;7(9). Order code M29-P.



Reviews
There are no reviews yet.