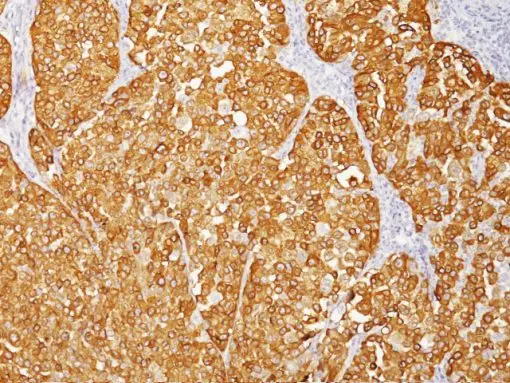
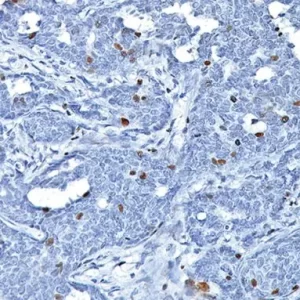
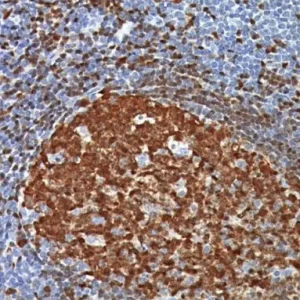
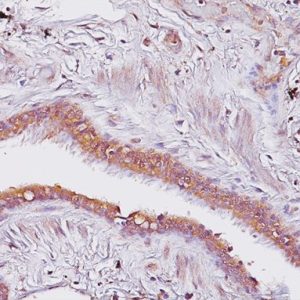
Description
The HMB45 clone reacts with a neuraminidase-sensitive oligosaccharide side chain of a glycoconjugate present in immature melanosomes. Studies have shown the HMB45-reactive antigen is present in cutaneous melanocytes, prenatal and infantile retinal pigment epithelium and melanoma cells. It is also thought to be oncofetal in nature (1). This antibody has been shown to label the majority of melanomas. The MART-1/Melan A recognizes a protein of 18kDa, identified as MART-1 (Melanoma Antigen Recognized by T cells 1) or Melan-A (1). Melan-A is a useful addition to melanoma panels which is specific to melanocytic lesions. Studies have also shown that MART-1 is more sensitive than HMB45 when labeling metastatic melanomas. Tyrosinase is a key enzyme involved in the initial stages of melanin biosynthesis. Studies have shown Tyrosinase to be a more sensitive marker when compared to HMB45 and MART-1. It has also been shown to label a higher percentage of desmoplastic melanomas than HMB45. The combination of HMB45, MART-1 cocktail and Tyrosinase make this quadruple antibody cocktail a first-order pan melanoma screener, and may prove to be a valuable marker for melanoma metastasis in sentinel lymph nodes (1,2).
SPECIFICATIONS
Specifications
| WEIGHT | N/A |
|---|---|
| DIMENSIONS | N/A |
| INTENDED USE | IVD |
| SOURCE | Mouse Monoclonal |
| CLONE | HMB45, M2-7C10 + M2 – 9E3, T311 |
| ISOTYPE | IgG1/Kappa, IgG2b + IgG2b, IgG2a |
| ANTIGEN | HMB45, MART-1, Tyrosinase |
| LOCALIZATION | Cytoplasmic |
DATASHEETS & SDS
REFERENCES
1. Orchard G. Evaluation of melanocytic neoplasms: application of a pan-melanoma antibody cocktail. Br J Biomed Sci. 2002;59(4):196-20.
2. Cook MG, Green MA, Anderson B, Eggermont AM, Ruiter DJ, Spatz A, Kissin MW, Powell BW; EORTC Melanoma Group.The development of optimal pathological assessment of sentinel lymph nodes for melanoma. J Pathol. 2003 Jul;200(3):314-9.
3. Center for Disease Control Manual. Guide: Safety Management, NO. CDC-22, Atlanta, GA. April 30, 1976 “Decontamination of Laboratory Sink Drains to Remove Azide Salts.”
4. Clinical and Laboratory Standards Institute (CLSI). Protection of Laboratory workers from occupationally Acquired Infections; Approved guideline-Third Edition CLSI document M29-A3 Wayne, PA 2005.


Reviews
There are no reviews yet.